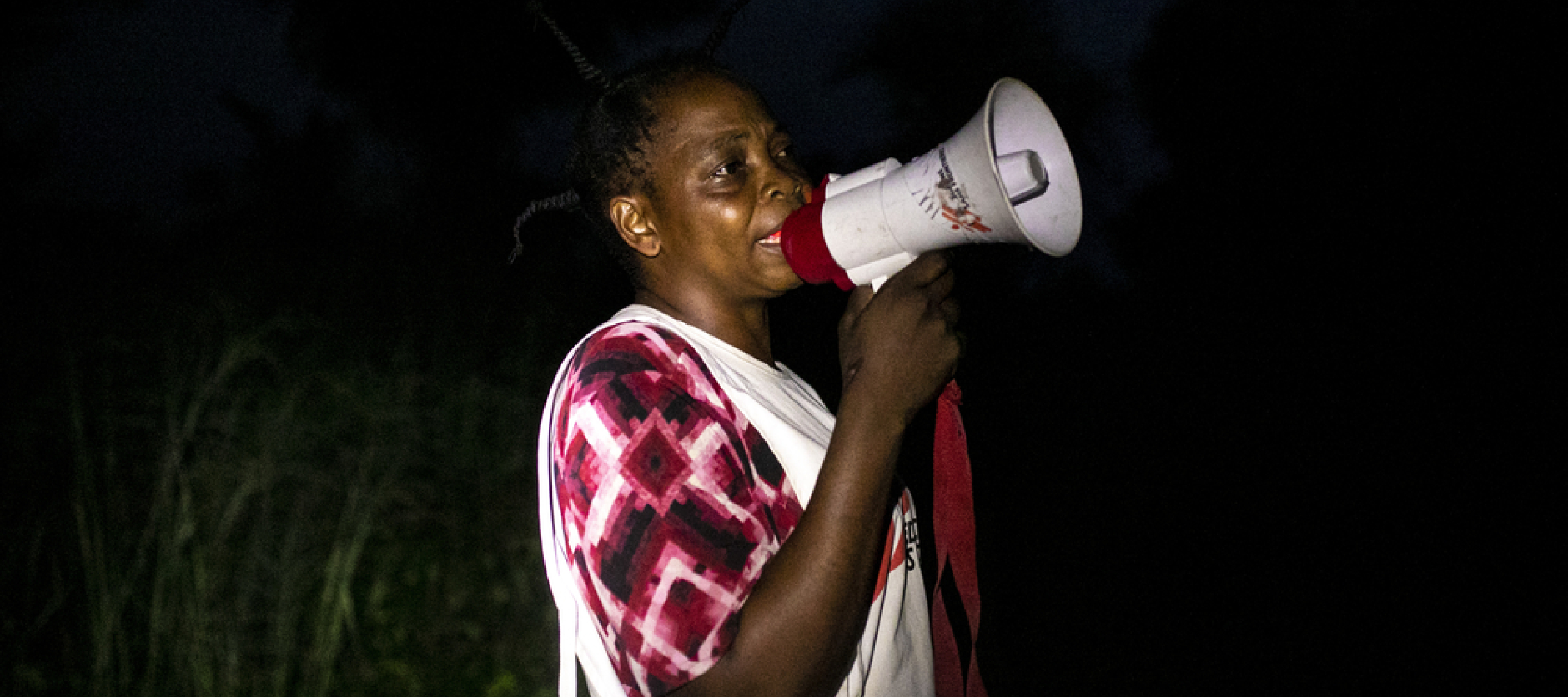
Eine Mitarbeiterin ruft mit einem Megafon die Bevölkerung auf, ihre Kinder zum Standort der Masernimpfung zu bringen.
Unsere Gesundheitspromoter beginnen ihre Arbeit vor Sonnenaufgang und arbeiten den ganzen Tag über, um sicherzustellen, dass die Nachricht bis zum letzten Haus der Gesundheitszone ankommt.
Unsere Gesundheitspromoter beginnen ihre Arbeit vor Sonnenaufgang und arbeiten den ganzen Tag über, um sicherzustellen, dass die Nachricht bis zum letzten Haus der Gesundheitszone ankommt.
Aktuelles und Berichte
Unsere Berichte informieren Sie ausführlich über die Aktivitäten und Projekte von Ärzte ohne Grenzen.
-
 |Haiti
|HaitiHaiti: Trotz alltäglicher Gewalt kämpfe ich für die Gesundheit der Patient*innen
Unsere Ärztin Priscille Cupidon setzt sich in Port-au-Prince trotz andauernder Gewalt und eskalierender Konflikte für die Gesundheit der Patient*innen ein.Blog -
 |Italien19. 04. 2024
|Italien19. 04. 2024Seenotrettung: Ermittlungen gegen Ärzte ohne Grenzen eingestellt
Nach sieben Jahren falscher Anschuldigungen wurde das Verfahren gegen Organisiationen, die Menschen in Seenot retten in Trapani in Sizilien, eingestellt.Presseaussendung -
 |Sudan12. 04. 2024
|Sudan12. 04. 2024Sudan: Ärzte ohne Grenzen fordert rasche Ausweitung von humanitärer Hilfe
Nach einem Jahr Krieg herrscht im Sudan eine der weltweit schwersten Krisen vergangener Jahrzehnte. Kriegsparteien behindern absichtlich die humanitäre Hilfe.Presseaussendung -
 |10. 04. 2024
|10. 04. 2024Ärzte ohne Grenzen kritisiert Asyl- und Migrationspakt der EU
Durch den Asyl- und Migrationspakt der EU wird sich die humanitäre Situation von Schutzsuchenden an den EU-Außengrenzen weiter verschlechtern.Presseaussendung -
 |28. 03. 2024
|28. 03. 2024Initiative gegen Infektionskrankheiten: Globale Petition für Preissenkungen medizinischer Tests gestartet
Petition: Ziel ist die Preissenkung von GeneXpert-Tests der Unternehmen Cepheid und Danaher. Ein niedriger Preis würde eine Diagnose für mehr Menschen bedeuten.Presseaussendung -
 |Libyen20. 03. 2024
|Libyen20. 03. 2024Mittelmeer: Ärzte ohne Grenzen kritisiert lebensgefährliche Manöver von libyscher Küstenwache
Die libyschen Küstenwache führte einen Pushback außerhalb ihres Zuständigkeitsbereichs durch, der das Leben von Hunderten Schutzsuchenden gefährdete.Presseaussendung -
 |Honduras
|HondurasWenn die Mücke sticht: Dengue-Fieber auf dem Vormarsch
In Honduras breitet sich Denuge aus. Innovative Methoden gegen das Virus sind gefragt, denn Klimawandel und Landflucht begünstigen die Brut der Mücken.Blog -
 |18. 03. 2024
|18. 03. 2024Zehn Jahre nach Ebola-Epidemie: Ärzte ohne Grenzen fordert Notvorrat an Medikamenten
Inzwischen gibt es zwei Impfstoffe, die – neben der Behandlung – für die Prävention und Reaktion auf einen Ebola-Ausbruch unerlässlich sind.Presseaussendung -
 |Tschad18. 03. 2024
|Tschad18. 03. 2024Sudan-Krieg: Hepatitis-E-Ausbruch in Geflüchtetencamps im Tschad
Wenn wir nicht schnell die hygienische Infrastruktur und den Zugang zu sauberem Wasser verbessern, riskieren wir einen Anstieg vermeidbarer Krankheiten sowie Todesfälle.Presseaussendung -
 |Nigeria12. 03. 2024
|Nigeria12. 03. 2024Nigeria: Humanitäre Krise im Nordwesten erfordert dringende Aufmerksamkeit
In Nigeria wird ein katastrophales Ausmaß an Mangelernährung und vermeidbaren Krankheiten registriert. Auf die vernachlässigte Krise muss reagiert werden.Presseaussendung -
 |Haiti06. 03. 2024
|Haiti06. 03. 2024Haiti: Ärzte ohne Grenzen verstärkt medizinische Hilfe nach drastischem Anstieg von Verletztenzahlen
Ärzte ohne Grenzen weitet die medizinische Hilfe in Haiti aus. Die Gewalt und die Verletztenzahlen steigen seit der Verschiebung der Parlamentswahlen stark.Presseaussendung -
 |Panama02. 03. 2024
|Panama02. 03. 2024Panama: Ärzte ohne Grenzen fordert Maßnahmen zur Eindämmung der Gewalt
Ärzte ohne Grenzen registriert einen Anstieg von gewaltsamen Übergriffen auf der Fluchtroute durch den Darién-Dschungel zwischen Kolumbien und Panama.Presseaussendung -
 |
|EU-Migrationspolitik: "Wir dürfen uns nicht an die Gewalt gewöhnen."
Bei unseren Einsätzen an den EU-Außengrenzen hören wir Geschichten von Gewalt und Leid. Die Festung Europa wird ausgebaut, obwohl andere humanitäre Krise dringend Aufmerksamkeit brauchen.Blog -
 |26. 02. 2024
|26. 02. 2024Ärzte ohne Grenzen warnt vor weltweitem Mangel an Cholera-Impfstoff
Ärzte ohne Grenzen ist besorgt über den weltweiten Mangel an Cholera-Impfstoff. Derzeit werden in 16 Ländern Cholera-Ausbrüche gemeldet.Presseaussendung -
 |Palästinensische Gebiete22. 02. 2024
|Palästinensische Gebiete22. 02. 2024Gazastreifen: Zwei Angehörige eines Mitarbeiters von Ärzte ohne Grenzen getötet
Eine Unterkunft von Ärzte ohne Grenzen wurde von israelischen Streitkräften angegriffen. Zwei Angehörige eines Mitarbeiters wurden getötet.Presseaussendung -
 |Palästinensische Gebiete22. 02. 2024
|Palästinensische Gebiete22. 02. 2024UN-Weltsicherheitsrat: Ärzte ohne Grenzen fordert sofortigen Waffenstillstand in Gaza
In der heutigen Sitzung des UN-Sicherheitsrats forderte unser Generalsekretär Christopher Lockyear einen sofortigen und anhaltenden Waffenstillstand in Gaza.Presseaussendung -
 |22. 02. 2024
|22. 02. 2024Ärzte ohne Grenzen veröffentlicht Bericht zu Folgen der europäischen Migrationspolitik
Ärzte ohne Grenzen macht auf die unmenschlichen Praktiken an europäischen Grenzen aufmerksam. Die EU-Migrationspolitik lagert Gewalt gegen Flüchtende aus.Presseaussendung -
 |Palästinensische Gebiete20. 02. 2024
|Palästinensische Gebiete20. 02. 2024Gaza: Ärzte ohne Grenzen fordert Schutz von Patient*innen im Nasser-Krankenhaus
Im einst größten Krankenhaus kann niemand mehr behandelt werden. Patient*innen müssen evakuiert werden. Wir fordern einen Waffenstillstand.Presseaussendung -
 |09. 02. 2024
|09. 02. 2024Ebola-Impfung halbiert Sterblichkeit bei infizierten Personen
Eine Studie der epidemiologischen Abteilung von Ärzte ohne Grenzen hat gezeigt, dass die Ebola-Impfung auch das Sterberisiko nach der Infektion halbiert.Presseaussendung -
 |Demokratische Republik Kongo08. 02. 2024
|Demokratische Republik Kongo08. 02. 2024D.R. Kongo: Hohe Zahl Verletzter durch Kämpfe in Nord-Kivu
Kämpfe bewaffneter Gruppen zwingen Tausende zur Flucht. Unsere medizinische Einrichtungen in Nord-Kivu versorgten zahlreiche Verletzte.Presseaussendung
